 |
|
|
 |
|
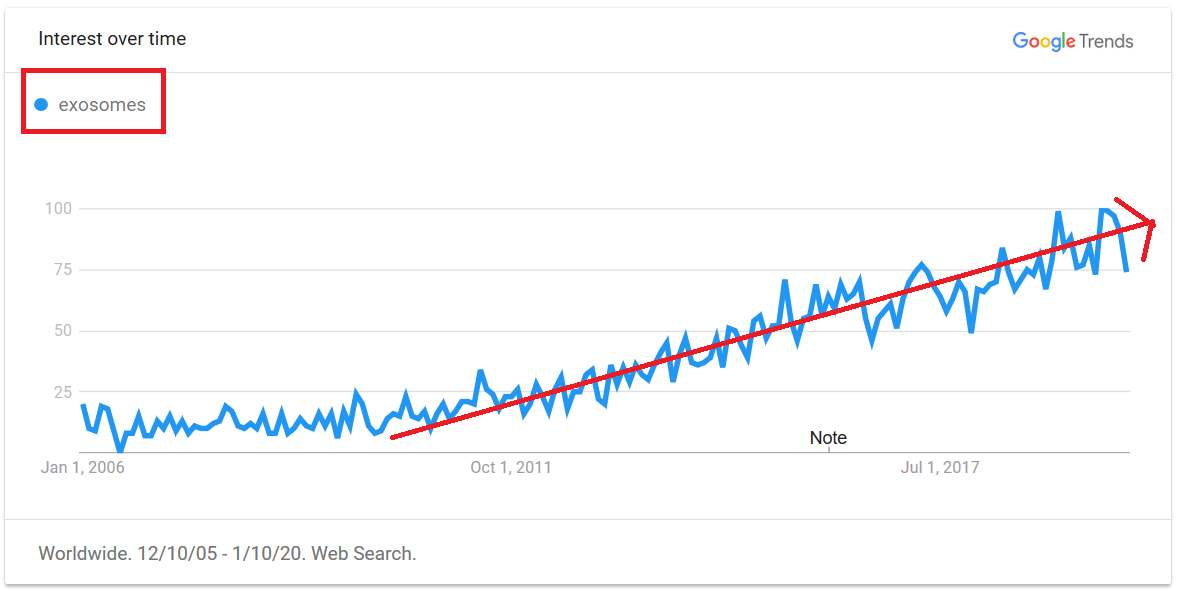
Exosomes, a Rising Star in Regenerative Medicine (Company Spotlights)
In recent years, exosomes have gained attention as a strategy for accessing the therapeutic effects of cells without the risks and difficulties of administering the cells to patients. With at least 12,431 scientific articles published about exosomes and 128 clinical trials underway, these small vesicles have intriguing potential to be used within a range of therapeutic applications. The term “exosomes” has become an increasingly common Search Term on Google over the past 10 years, as shown in the graph above.
|
|
|
|
6th Perinatal Stem Cell Society Congress, March 5-6, 2020 (Salt Lake City, UT)
Hosted by the Perinatal Stem Cell Society, this two-day event will feature the world’s leading perinatal stem cell researchers, specializing in Amnion, Amniotic Fluid, Cord Blood for Regenerative Medicine, Cord Tissue, and the Placenta. Its scientific program will cover all aspects of the perinatal stem cell field, with opportunities to learn from leading clinicians utilizing cellular therapies within their practices. Click here to learn more or register.
|
|
|
|
First Dosing in Plexoval Exosome Wound Healing Human Study
Regenerative medicine company Exopharm Limited (ASX:EX1) announces first dosing has occurred in the PLEXOVAL Phase I study using exosomes isolated from human platelets for wound healing, Exopharm’s first human clinical trial. Further participants are expected to soon follow in this study that involves up to 20 participants.
|
|
|
|
Aegle Therapeutics Raises $4M to Fund First Trial in the U.S. Using Exosomes from Allogeneic MSCs
Aegle Therapeutics Corp., a first in class biotechnology company isolating extracellular vesicles, including exosomes (“EVs”), secreted by mesenchymal stem cells as therapy, announced the closing of a $4M financing. Aegle’s platform technology is initially being developed to treat dystrophic epidermolysis bullosa (“DEB”), a rare pediatric skin blistering disorder. Aegle’s IND for the treatment of DEB patients has been cleared by the FDA. The Company anticipates beginning clinical trials in DEB in the first half of 2020.
|
|
|
|
American Journal of Sports Medicine to publish results of an FDA-approved clinical trial for treating knee pain
GID BIO announced the acceptance by the American Journal of Sports Medicine to publish results of an FDA-approved multi-site, randomized, placebo-controlled Phase IIb clinical trial measuring the safety and efficacy of its SVF-2 device and point-of-care (POC) therapy intended to treat pain and function associated with knee osteoarthritis. In a major development, the study was approved by the FDA under IDE and is the first regenerative cell therapy to meet endpoints using autologous stromal cells from adipose tissue.
|
|
|
|
INTELLiSTEM Announces the Launch of a New Antigen Identification Platform for the Development of Novel CAR T Cells
INTELLiSTEM Technologies, an international biotechnology company revolutionizing cell therapy, has announced the launch of IntelliPeptidome(TM), a novel Drug Discovery Platform for rapid and systematic identification of MHC/HLA bound peptide candidates for various clinical applications. With IntelliPeptidome(TM), Intellistem is launching its partnering program to work with a selective and distinctive CAR T cell companies in the field to target difficult solid tumors.
|
|
|
|
Professional Short Course on “Preservation of Cellular Therapies” by University of MN (May 18-19, 2020)
Preservation of Cellular Therapies is a professional short course to provide practical training for individuals involved in the preservation of cellular therapies. Come learn about fundamentals of preservation, protocol development, design of a storage facility, regulatory issues associated with preservation of cell therapies, clinical issues and more. The course is appropriate for manufacturing engineers, managers as well as technicians who work with cell-based products: cell banks, biobanks, companies that use cell-based assays, cell therapy companies, regenerative medicine companies, hospitals or cell therapy laboratories.
|
|
|
|
TreeFrog Therapeutics brings in senior profiles to drive the advent of high-quality & affordable allogenic cell therapies
TreeFrog Therapeutics, a pure player in iPSC-derived cell therapies, announces the appointment of 2 senior executives and 2 independent board members. Pascale Berthet, formerly Business Development Manager at FUJIFILM Cellular Dynamics was appointed Chief Business Development Officer, and Michaël Lanero Fidalgo, former Head of EMEA Biodevelopment Center at Merck KGaA, was recruited as Chief Operating Officer. Two independent board members also joined its corporate governance: Julia Berretta, formerly VP of Business Development at Cellectis, and Frederic Desdouits, former Bionest Partner and Pierre Fabre North America Director.
|
|
|
|
How Anemocyte is Changing History as a Biotech Manufacturing Organization (BMO)
Anemocyte is the world’s first Biotech Manufacturing Organization (BMO). The company’s Cell Therapy Platform supports the manufacturing of autologous and allogeneic products, its DNA Platform produces plasmid DNA for viral vector production, and its Gene Therapy Platform supports process development and implementation for non-viral gene modified cells. In this interview with Anemocyte’s CEO, Marco Ferrari, we explore the company’s role as a BMO, its investment into research and technology, and its future directions.
|
|
|
|
StemBioSys Acquires a Majority Stake in Cartox
StemBioSys, Inc. (StemBioSys) and CarTox, Inc. (Cartox) are pleased to announce that StemBioSys has acquired a majority stake in Cartox. StemBioSys will acquire all remaining equity of Cartox subject to the achievement of certain milestones. Cartox has developed an innovative approach to test cardiac safety of new drugs before those drugs are given to human patients in clinical trial programs.
|
|
|
|
Stem Cells in Drug Discovery: A Changing Paradigm
Drug attrition rates using animal models are usually high, because present preclinical assays do not reliably detect potential risk of damage to the heart, kidney, liver, and brain. The discovery of induced pluripotent stem cells (iPSCs) and achievements in producing these cells from patients and healthy individuals along with efficient gene modification has led to remarkable opportunities over the last several years to model human disease.
|
|
|
|
BMS Pays Evotec $6M as Companies Expand iPSC Collaboration
Evotec SE announced that the Company received a US$ 6 million payment from Bristol-Myers Squibb Company (NYSE:BMY) following the decision to expand the collaboration to include additional cell lines. Evotec and Celgene, which is now a Bristol-Myers Squibb company, initiated the collaboration in December 2016 to identify disease-modifying treatments for a broad range of neurodegenerative diseases.
|
|
|
|
Mesenchymal Stem Cells (MSCs) in the Era of Regenerative Medicine
Mesenchymal stem cells are a class of multipotent cells found in human tissues, such as bone marrow, adipose tissue, dental pulp, and periosteum, a layer of connective tissue that envelops the bones. They are also known as: Medicinal Signaling Cells, Mesenchymal Stromal Cells, and Marrow Stromal Cells. Given this naming variability, as well as urging by Dr. Caplan to rename the cells so that patients don’t assume specific medical benefits, these cells are best known as: MSCs.
|
|
|
 |
|
|
|
Global Database of Exosome Companies (2020)
This is a searchable, sortable database that reveals the identities of companies worldwide who are developing exosome therapies. It takes our analysts countless hours of research to uncover exosome companies from across the globe, identifying their products, technologies and partnerships/co-development programs. This database features companies who are developing exosome and extracellular vesicle (EV) therapies and the platform technologies to support them.
|
 |
|
|
|
|
|
|