 |
|
|
|
|
 |
|
|
Induced Pluripotent Stem Cell (iPSC) Industry Trends Dominating in 2025
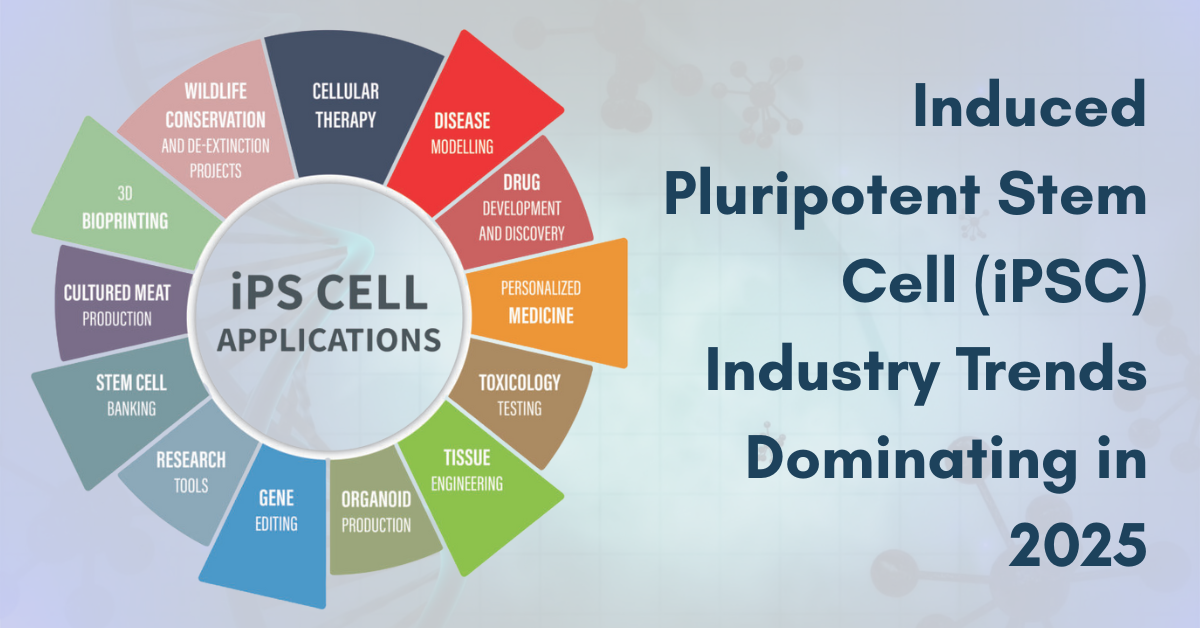
Today, iPSCs are being explored for applications related to drug screening, toxicological studies, disease modeling, cell therapy, personalized medicine, cultured meat production, and beyond. Of course, the world’s largest research supply companies are also commercializing a diverse range of iPSC-derived products and services. This article explores the various iPSC industry trends that are dominating today in 2025 and the leading market competitors within each niche. |
|
|
Downstream Processing Solutions for Extracellular Vesicle Programs
Scalable extracellular vesicle purification requires complex equipment, expertise, and investment in process development. Take advantage of RoosterBio's industry-leading expertise and optimized processing solutions instead with QuickShip™ DSP. QuickShip DSP takes conditioned media and rapidly processes it into vials of either concentrated EVs/enriched secretome or purified EVs, enabling faster results without needing to acquire complex downstream processing capabilities.
|
|
|
 |
|
|
Ncardia Launches First-of-Its-Kind iPSC-Derived Non-Human Primate Cardiomyocytes to Advance Cardiac Safety Research
September 24, 2025, LEIDEN, Netherlands — Ncardia, a leader in stem cell–based solutions for drug discovery and safety assessment, today announced the launch of Ncyte® NHP-C vCardiomyocytes, the first commercially available ventricular-like cardiomyocytes derived from induced pluripotent stem cells (iPSCs) of cynomolgus monkeys. New Ncyte® NHP-C vCardiomyocytes provide a scalable, ethical and translational tool for cross-species cardiac studies. |
|
|
Mytos Advances Automated iPSC Manufacturing through Strategic Partnerships with StemSight, Rinri Therapeutics, and Novadip
September 3, 2025, London, England — Mytos, a biotechnology company automating cell manufacturing, announced new manufacturing collaborations with three innovative biotech companies – StemSight, Rinri Therapeutics, and Novadip. Each company intends to integrate Mytos’ iDEM automated cell manufacturing platform to scale the production of diverse cell types for therapeutic use, highlighting the platform’s versatility to create cells to treat ocular diseases, hearing loss, and bone defects. |
|
|
Take Your Biotherapies Expertise to the Next Level! Free CABP Exam Prep Bootcamps Series From AABB
Provided by the Association for the Advancement of Blood & Biotherapies (AABB), the CABP is a pioneering credentialing exam, identifying high-caliber professionals in cell and gene therapy. Becoming a CABP is a mark of distinction contributing to the advancement of the field by demonstrating a high standard of competence across key domains of knowledge. AABB has introduced an exam prep bootcamps series supporting candidates on their journey to earning this prestigious credential, with each expert-developed bootcamp covering one of the CABP exam domains.
Bonus offer: For complimentary access to the full bootcamp series usually worth $275, AABB encourages candidates to submit a CABP application (subject to approval). Learn more about the CABP, the bootcamp series and how to apply on the AABB website. |
|
|
Takara Bio Europe launches Cellartis® MSC EV Wonder™, a defined, xeno-free medium for high-yield EV production
September 24, 2025 – Takara Bio Europe announces the launch of Cellartis® MSC EV Wonder™, a defined, xeno-free medium engineered for the scalable production of extracellular vesicles (EVs) from human mesenchymal stem cells (MSCs). MSC-derived EVs are increasingly recognized as next-generation, cell-free therapeutic candidates, offering regenerative and immunomodulatory benefits without the safety concerns associated with administering intact MSCs. As the field shifts from MSC-based approaches to MSC-EVs, researchers are seeking solutions that deliver both robust yields and reproducible quality at scale. |
|
|
Cartherics Wins ‘Most Promising iPSC Therapy Pipeline in APAC’ at Asia Pacific Cell & Gene Therapy Excellence Awards
Melbourne, Australia, 11 September 2025 – Cartherics Pty Ltd (“Cartherics” or “Company”), a biotechnology company developing off-the-shelf immune cell therapies focusing on high-impact women’s diseases, with lead programs in ovarian cancer and endometriosis, is proud to announce that it has been named ‘Most Promising iPSC Therapy Pipeline in APAC’ in the Asia Pacific Cell & Gene Therapy Excellence Awards 2025 (APCGTEA 2025). |
|
|
 |
|
|
AABB’s Cell and Gene Therapy Standards for Pharmacy, First Edition, Takes Effect October 1
Sept 8, 2025, Bethesda, MD – The Association for the Advancement of Blood & Biotherapies (AABB) is pleased to announce the release of the first edition of Cell and Gene Therapy Standards for Pharmacy, which take effect Oct. 1, 2025, and are now available for purchase on the AABB Store. These new Standards represent a critical advancement in strengthening quality and safety for cell and gene therapy (CGT) products managed by pharmacies. |
|
|
Cell & Gene Therapy Developers Need an Excipient-Certified Cell Culture Media Manufacturer
In cell and gene therapy, product safety and consistency are non-negotiable. Every component in the manufacturing process—including cell culture media—directly impacts product quality, patient safety, and regulatory success. That’s why using an excipient-certified cell culture media manufacturer is critical. Explore why Nucleus Biologics, the first excipient-certified custom media provider, is a trusted partner for excipient-grade solutions.
Excipient certification confirms that the manufacturer adheres to stringent quality standards for raw materials, documentation, and process controls. This rigorous certification reduces risks from contaminants or batch variability, ensuring consistent performance in sensitive applications. It also provides full traceability and aligns with advanced regulatory expectations, streamlining compliance for critical development stages. |
|
|
Sanatela AT Medical Solutions Inc. Announces Groundbreaking Patent Issuance for Cancer Stem Cell Identification and Precision Treatment Platform
Rochester, NY, September 24, 2025 – After two decades of pioneering hematology and cancer stem cell research, Sanatela AT Medical Solutions Inc. proudly announces that the United States Patent and Trademark Office (USPTO) has officially issued its long-awaited patent for a revolutionary cancer detection and treatment platform. |
|
|
Regenerelle® Issues Response to FDA Untitled Letter
September 8, 2025, Rochester, NY — Regenerelle®, a global leader in the manufacturing and distribution of Wharton’s Jelly Mesenchymal Stromal Cells (WJ-MSCs) and exosomes derived from WJ-MSCs, today announced the public release of its full response to the U.S. Food and Drug Administration (FDA) Untitled Letter dated May 22, 2023. |
|
|
European Commission Grants First Approval for Expanded Cord Blood Stem Cell Therapy (Zemcelpro®)
September 10, 2025, Montreal, Canada — In a landmark move for regenerative medicine, the European Commission has conditionally authorized Zemcelpro®, the first treatment in Europe to use expanded umbilical cord blood stem cells. Developed by Canadian biotech company ExCellThera, this therapy is only the second of its kind approved worldwide and represents a major advance in transplant options for patients with blood cancers such as leukemias and myelodysplastic syndromes. |
|
|
Congress Moves to Reauthorize Funding for National Cord Blood and Bone Marrow Transplant Programs
A bipartisan group of lawmakers in the U.S. House has introduced new legislation to extend funding for two cornerstone programs that connect patients with life-saving stem cell treatments. The "Stem Cell Therapeutic and Research Reauthorization Act of 2025" proposes more than $280 million in federal funding over the next five years, ensuring that these initiatives continue past their current 2026 expiration date. |
|
|
CytoMed Expands NK cells from Stored Cord Blood, Creates to New Cord Blood-Derived Biotech Under LongevityBank
SINGAPORE, Aug. 28, 2025 — CytoMed Therapeutics Limited (NASDAQ: GDTC) (“CytoMed”), a Singapore-based clinical stage biopharmaceutical company focused on harnessing its proprietary technologies to develop novel affordable donor-derived cell-based immunotherapies for the treatment of a broad range of cancers, including both blood and solid tumours, has successfully expanded clinical-scale natural killer (“NK”) cells from cord blood units that have been cryopreserved for more than a decade. This sets the impetus for establishing a new business platform to generate additional revenue streams and future growth. |
|
|
Shineco Establishes Biological Cell Digital Division to Integrate iPSC Technology Assets with Blockchain Technology
BEIJING, Aug. 13, 2025 — Shineco Inc. (NASDAQ: SISI), a manufacturer of induced pluripotent stem cell (iPSC) technology platforms, announced the establishment of a Biological Cell Digital Business Division (the “Division”), and the appointment of Mr. Lin Hongguang as General Manager, to advance the integration of its biological cell assets and blockchain technology. Through a new architecture of blockchain-based cell assets, Shineco aims to build a vertical ecosystem within the biological cell sector, accelerate the global distribution of biological cell products and boost global sales. |
|
|
Historic Patent Challenges to Pluripotent Stem Cells (ESCs and iPSCs)
The patent landscape for pluripotent stem cells, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), is characterized by groundbreaking discoveries, legal challenges, and debates over accessibility. The patent environment surrounding these stem cell types is complicated, and there are a number of restrictions on how the cells can be used for commercial and strategic purposes. |
|
|
Cord Blood Industry Consolidation Escalates in 2025
The global cord blood industry experienced strong growth from the early 1990’s through the late 2000’s, during which time more than 450 cord blood banking companies emerged worldwide. Approximately half of those companies owned their own labs, while the others were marketing entities or sub-contractors of storage space. Starting about ten years ago, the market suddenly changed course. It entered a period of aggressive consolidation, cutting the global number of cord blood banks by approximately half. |
|
|
RMAT Momentum Continues: Publicly Announced Designations Rises to 146
To date, what number of U.S. FDA-approved RMATs have been issued and to whom have they been awarded? The answer is that 146 RMAT (Regenerative Medicine Advanced Therapy) designations have been publicly announced by biotech and pharma companies. However, the FDA states it has received 368 requests and issued 184, which means that a handful are not yet public knowledge. Therefore, a few companies are operating in stealth mode with regard to their RMAT designations and half (50%) of RMAT applications get approved (184 approvals / 368 applications = 50.0%). |
|
|
 |
|
|
[FLASH SALE] Mesenchymal Stem Cells / Medicinal Signaling Cells (MSCs) – Advances & Applications, 2025
Today, 12 MSC-based products have received regulatory approvals. This includes 11 full approvals and a 12th conditional approval within China. The Republic of Korea has approved five products; Japan and the EU have approved two products; and India, Iran, and Australia have approved one each. Market competitors have also developed 17 biomaterial-based MSCs and MSC progenitor products, which are largely being used for orthopedic indications. Companies like Cynata are pioneering iPSC-derived MSC (iMSC) production technologies, supporting large-scale therapeutic development. In total, at least eight companies are now developing iMSCs.
For 48 hours only, you can claim this global strategic report for 20% off. |
|
|
[FLASH SALE] Global Induced Pluripotent Stem Cell (iPSC) Industry Report – Market Size, Trends, & Forecasts, 2025
In addition to companies developing iPSC-derived cellular therapeutics, a growing number of competitors are commercializing iPSC products for diverse applications—ranging from drug discovery and disease modeling to toxicology testing, personalized medicine, tissue engineering, 3D bioprinting, and clean meat production. This report presents key industry metrics, including scientific publications, clinical trials, patents, funding rounds, strategic partnerships, and M&A activity. It identifies major global iPSC market players and provides detailed market size data segmented by Application, Technology, Cell Type, and Geography (North America, Europe, Asia-Pacific, and RoW), along with projections through 2030.
For 48 hours only, you can claim this global strategic report for 20% off. |
|
|
[UPDATED FOR 2026] Global Regenerative Medicine (RM) Industry Database, 2026 - Featuring 1,920+ Companies
Regenerative medicine (RM) companies are continuing to multiply. There are now an astonishing 1,920+ companies developing RM products to advance human health. This database is designed to help you identify all known market participants in this fast-growing field, including competitors, collaborators, or acquisition targets. RM companies include those advancing stem cell therapies, cell and gene therapies, tissue engineering and biomaterials, direct cell reprogramming, exosome therapeutics, cellular scaffolds and matrices, 3D bioprinting, and more.
Because this database was just updated, for a limited time, you can claim it for an $150 off. |
|
|
Interested to learn about other market segments, such as MSCs, iPSCs, CAR-T cells, exosomes, cord blood, or the cell therapy industry at large?
To view all available products, explore the BIOINFORMANT SHOP. |
|
|
*Want to advertise in this newsletter? Contact us at Info@BioInformant.com. |
 |
|
|
|
|
|
|
|
|