 |
|
|
|
|
 |
|
|
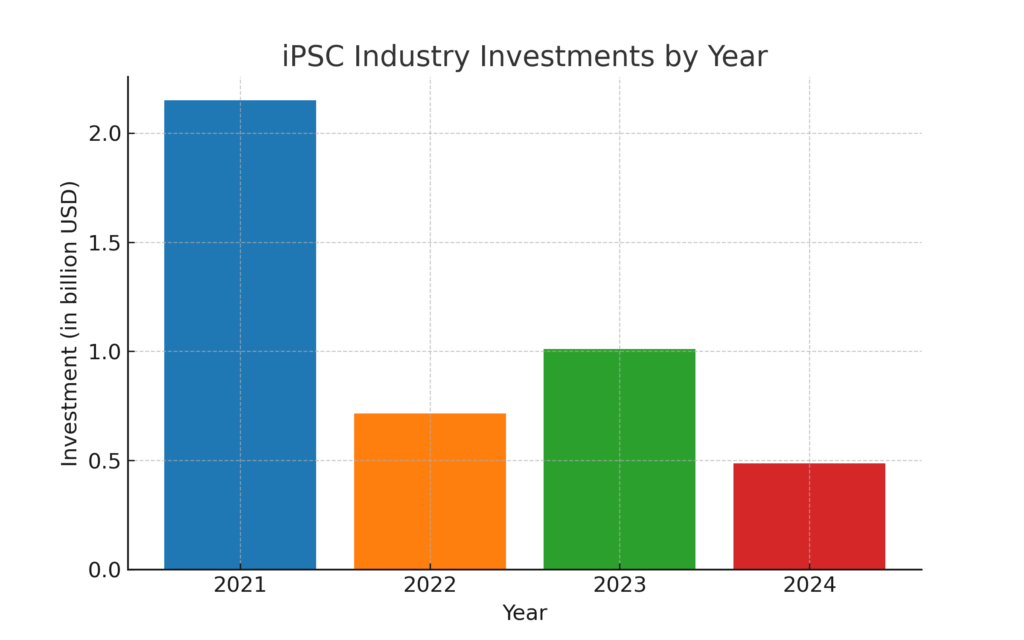
Venture Capital Funding Flowing into the iPSC Sector by Year
The induced pluripotent stem cell (iPSC) sector has attracted strong venture capital funding in recent years, reflecting growing confidence in its potential across regenerative medicine, drug development, toxicology testing, personalized medicine, and disease modeling. From 2021 to 2024, numerous companies secured substantial investments, signaling a promising future for iPSC-based innovations. 2021 was a particularly strong year, with iPSC industry investments totaling over $2.15 billion. |
|
|
Access Billions of Xeno-Free hMSC-derived Exosomes for Your R&D Program
Manufactured with a combination of best-in-class cells and downstream processing, RoosterVial™ Exosomes provide you with access to highly characterized exosomes without needing to invest resources to manufacture them in your lab. These vialed exosomes collected from xeno-free human umbilical cord-, bone marrow-, and adipose-derived MSCs are ready to deliver immediate results in your research and development programs. |
|
|
How CellPort Is Making Cell Culture Smarter – Interview with Patrick Dentinger, CEO of CellPort Software
In the dynamic world of cell therapy, where precision and reproducibility are paramount, one scientist’s frustration with cell unpredictability sparked an industry-changing innovation. After years at the bench wrestling with the inherent variability of cell culture, Patrick Dentinger recognized a deeper problem—labs weren’t equipped with the right tools to track, understand, and manage cellular drift. |
|
|
I Peace Launches Personalized iPS Cell Services and Longevity Treatment in the U.S. – Age-Reversed Stem Cell Technology Now Available to the Public
Palo Alto, CA, April 25, 2025 – I Peace, Inc., a global pioneer in iPSC technology, proudly announces the launch of its personalized iPS cell production and longevity-focused rejuvenation therapies in the United States. I Peace CEO Koji Tanabe spoke at the “Unlimited Health Conference,” a part of the Vitalist Bay, the biggest longevity event in the world, which is held in Berkeley, California. The event was held on April 5. |
|
|
 |
|
|
The Future of Allogeneic Cell Therapy is Off-the-Shelf and iPSC-Based – An Interview with Cellistic’s Founder and CTO, Stefan Braam, PhD
In the rapidly evolving field of cell therapy, scalability and accessibility remain two of the biggest challenges for widespread patient impact. Cellistic, a leading company that is pioneering allogeneic cell therapy solutions, is tackling these obstacles head-on with its novel iPSC technologies. In this interview, Stefan Braam, PhD, Founder & CTO of Cellistic, shares insights into the company’s journey, its innovative platforms—including Pulse™, Echo™, and the newly introduced Allo Chassis™—and how these advancements are poised to redefine cell therapy development as we known it. |
|
|
Set yourself apart by becoming a Certified Advanced Biotherapies Professional (CABP) in 2025!
Provided by the Association for the Advancement of Blood & Biotherapies, the CABP is a pioneering credentialing exam, designed to identify high-caliber professionals working in cell and gene therapy. Earning the CABP is a mark of distinction that elevates your career to the next level, gives you a wider perspective of the field, helps you work better with others and makes for more powerful teams.
There is no better time to set yourself the goal of becoming a CABP in 2025 with this bonus offer: AABB is pleased to introduce a video series of exam prep bootcamps designed to support candidates on their journey to earning this prestigious credential. To receive complimentary access to the full bootcamp series usually worth $275, AABB encourages candidates to submit a CABP exam application (subject to approval) - learn more about the CABP exam, the new bootcamp series and how to apply on the AABB website. |
|
|
RoosterBio Announces Collaboration with Thermo Fisher Scientific to Advance Cell and Exosome Therapy Manufacturing
FREDERICK, MD, April 29, 2025 — RoosterBio, Inc., a leading supplier of adult human mesenchymal stem/stromal cells (hMSCs), highly engineered media, and bioprocess development services, announced a new collaboration with Thermo Fisher Scientific, the world leader in serving science. This collaboration aims to accelerate the availability of new, potentially life-saving cell and exosome therapies that have the potential to revolutionize the treatment of degenerative disease. |
|
|
Curi Bio and Cook MyoSite Announce Strategic Partnership
SEATTLE & PITTSBURGH, April 30, 2025 — Curi Bio, the global leader in human 3D engineered tissue platforms, and Cook MyoSite, a pioneer in the development of skeletal muscle-based therapies, today announced a strategic partnership to develop advanced, predictive human engineered tissue models aimed at addressing muscle-related diseases and metabolic disorders, including obesity and diabetes. |
|
|
 |
|
|
Nikon CeLL Innovation Enters into a Strategic License Agreement with RoosterBio, a US Company, to Enable GCTP/GMP Manufacturing Platform of Human Mesenchymal Stem Cells & Extracellular Vesicle in Japan
TOKYO, Japan, April 15, 2025 – Nikon CeLL innovation Co., Ltd. (NCLi), a subsidiary of Nikon Corporation has entered into a strategic licensing agreement with RoosterBio, a leading stem cell technology company in the US. This agreement provides the Japanese biopharma industry with an end-to-end solution for development and manufacturing of human MSC and extracellular vesicle (EV) therapeutics. Drug developers can leverage RoosterBio’s technology platform to accelerate the development of their advanced therapies with NCLi, and then seamlessly transition to clinical manufacturing within NCLi’s GCTP*1/GMP*2 facility designed specifically for cell therapies. |
|
|
Cellistic Launches Echo™ – NK Platform, a Scalable Solution for Off-the-Shelf Allogeneic Immune Cell Therapies
Belgium, Mont Saint-Guibert, April 10, 2025 – Cellistic, a pioneer in iPSC-based cell therapies, is launching the Echo™-NK platform to enable the scalable manufacturing of allogeneic cell therapies to target multiple diseases. Natural Killer (NK) cells are increasingly leveraged in these therapies for their ability to destroy harmful cancerous cells in their early stages, making them a promising option for the treatment of blood cancers, solid tumors, and autoimmune diseases. Cellistic’s Echo™-NK platform provides drug developers with a commercially viable solution for an Off-the-Shelf NK cell therapy. |
|
|
PeptiGrowth Inc. is Launching a Novel Synthetic Peptide: KGF alternative peptide (FGFR2b agonist)
Tokyo, Japan, April 1, 2025 - PeptiGrowth Inc. (Headquarters: Chiyoda-ku, Tokyo, President: Junichiro Ishizuka) has successfully developed a novel synthetic peptide called “KGF alternative- peptide (FGFR2b agonist)” which is functionally equivalent to recombinant keratinocyte growth factor (KGF). This product entered the market in April 2025. |
|
|
Altaris Launches Minaris Advanced Therapies, Merging the CDMOs Minaris Regenerative Medicine and WuXi Advanced Therapies
In a bold move that has consolidated the landscape of advanced therapy manufacturing, Altaris LLC announced the successful acquisition of two key cell and gene therapy operations—Advanced Therapies U.S. and OXGENE—from WuXi AppTec. Completed on March 7, 2025, the transaction marks a strategic expansion of Altaris’ footprint in the fast-growing cell and gene therapy contract development and manufacturing organization (CDMO) space. |
|
|
Sernova Advances Cell Pouch Type I Diabetes Trial; Will Test iPSC-Derived Clusters Next
LONDON, ONTARIO, and BOSTON, April 07, 2025 — Sernova Biotherapeutics, a leading RM company focused on developing it’s Cell Pouch Bio-hybrid Organ as a functional cure for type 1 diabetes (T1D), provided an update on its ongoing clinical trial in patients with T1D. Yesterday, on March 31, the Data and Safety Management Board (DSMB) for Sernova’s Phase 1/2 clinical trial, conducted a scheduled annual data review. The DSMB has sanctioned the enrollment of the final patient in Cohort B. |
|
|
LIfT BioSciences gains access to half a million allogeneic donors through collaboration with Gift of Life Biologics
London, 03 April 2025 – LIfT BioSciences, (‘LIfT’ or ‘the Company’), a rapidly emerging biotech and the global leader in neutrophil immunotherapies, and Gift of Life Biologics, (‘GOLB’) a leader in the provision of cellular starting material for cell therapies, announce a collaboration with GOLB as LIfT’s preferred partner for sourcing hematopoietic stem cells (HSCs) for the production of its clinical grade Immunomodulatory Alpha Neutrophils (IMANs), a first-in-class cell therapy designed to overcome treatment resistance in solid tumours as the Company prepares to initiate its clinical trials. |
|
|
New York Blood Center Enterprises and Human Life CORD Sign Letter of Intent to Expand Manufacturing Collaboration for Umbilical Cord -Derived MSCs
NEW YORK, March 25, 2025 — New York Blood Center Enterprises (NYBCe) and Human Life CORD Japan Inc. (Human Life CORD) have signed a Letter of Intent to expand their manufacturing collaboration for mesenchymal stromal cells (MSCs) through Comprehensive Cell Solutions (CCS), a business unit of NYBCe. This strategic partnership aims to drive innovation and enhance the global supply chain for next-generation umbilical cord tissue-derived MSCs.
|
|
|
RoslinCT and Ayrmid Pharma Enter Into a Strategic Partnership to Manufacture FDA Approved Cell Therapy Omisirge® (omidubicel-onlv)
BOSTON, MA, Mar 20, 2025 — Ayrmid Pharma Ltd., a cell therapy pioneer working to turn cells into powerful therapeutics and RoslinCT, a world leading cell and gene therapy CDMO, announced their intent to enter into a strategic partnership for the production of Omisirge, a commercial product for the treatment of hematologic malignancies commercialized in the US by Gamida Cell Inc, a subsidiary of Ayrmid Pharma Ltd. |
|
|
Garuda Therapeutics Closes $50 Million Series A-1 Financing to Advance Off-the-Shelf Blood Stem Cell Therapies
CAMBRIDGE, MA, March 04, 2025 — Garuda Therapeutics, a biotech company creating off-the-shelf, HSC therapies for a multitude of blood disorders, announced the successful completion of an approximately $50 million Series A-1 funding round. Investors included OrbiMed, Northpond Ventures and Cormorant Asset Management, with new strategic investment from Kyowa Kirin Co., Ltd., a Japan-based Global Specialty Pharmaceutical Company. |
|
|
The Remarkable Rise of iPSC-derived MSC Therapeutics: iMSCs
The rise of iPSC-derived mesenchymal stem cells (iMSCs) represents a significant evolution in regenerative medicine. iMSCs are generated by reprogramming adult somatic cells, typically fibroblasts, into induced pluripotent stem cells (iPSCs), which are then differentiated into mesenchymal stem cells (MSCs). This breakthrough technology offers several advantages over traditional MSC therapies, which are often derived from limited, patient-specific sources such as bone marrow or adipose tissue. |
|
|
What Is An RMAT? List of Publicly Announced RMAT Designations (131)
To date, what number of U.S. FDA-approved RMATs have been issued and to whom have they been awarded?. The answer is that 131 RMAT (Regenerative Medicine Advanced Therapy) designations have been publicly announced by biotech and pharma companies. However, the FDA states it has received 317 requests and issued 151, which means that a handful are not yet public knowledge. Therefore, a few companies are operating in stealth mode with regard to their RMAT designations and approximately 47.6% of RMAT applications get approved (151 approvals / 317 applications = 47.6%). |
|
|
REPROCELL’s Clinical-Grade iPSCs: Advancing Cell Therapy
In the rapidly evolving field of regenerative medicine, REPROCELL’s StemRNA™ Clinical iPSCs provide a benchmark for clinical grade induced pluripotent stem cells (iPSCs). These high-quality iPSCs provide a foundation for various cell therapy programs, ensuring safety, consistency, and regulatory compliance. Our clinical iPSCs are currently being evaluated by many biopharma companies globally, are selected for regulatory filings and are IND approved. REPROCELL is a trusted partner for cell therapy developers who are seeking robust, high-quality clinical iPSC banks that meet industry standards. |
|
|
The Rise of Exosome-Based Cosmeceuticals in 2025
A cosmeceutical is a product that is marketed as a cosmetic but has the capacity to exert pharmaceutical effects. In recent years, exosomes have been utilized within the global cosmetics industry to manufacture and market skincare products that use the power of these small extracellular vesicles to enhance a broad range of traits, including but not limited to skin health and rejuvenation, scar improvement, hair regrowth, anti-aging effects, and beyond. Exosomes can provide bioactive substances such as proteins, lipids, and nucleic acids to the target cells to alter gene expression and cellular function. Importantly, exosomes can also induce collagen formation, enhance tissue regeneration, and exert anti-inflammatory properties. |
|
|
The Role of iPSCs in Farm Animals and Wildlife Conservation
Since their discovery in 2006, iPSCs have been potently transforming regenerative medicine, biotechnology, and conservation. Their ability to be reprogrammed from adult somatic cells unlocks new possibilities for enhancing farm animal reproduction, boosting disease resistance, and improving food quality, while also playing a pivotal role in wildlife conservation and species revival. However, their use raises ethical concerns, including the risks of genetic modification, unforeseen ecological consequences, and the moral dilemmas surrounding species de-extinction. |
|
|
How Human iPSCs are Revolutionizing Drug Discovery in 2025
Drug discovery involves high cost and uncertain outcomes. Only a few companies can take the risk of investing enormous amounts of money into research and testing, only to see undesirable side effects emerge during the final human clinical trials. In many cases, companies are spending millions of dollars to fail during clinical testing. Thankfully, some new medicines are now being tested for safety on specialized cells developed from human pluripotent cell lines. Cell types which are increasingly being used within drug discovery applications include heart (cardiomyocytes) and liver cells (hepatocytes), which are the organs where 80% of drug failures occur. |
|
|
CAR-T Cell Companies Proliferate: List of CAR-T Companies Worldwide
CAR-T cell therapy is a type of immunotherapy that teaches T cells to recognize and destroy cancer. This article provides a comprehensive list of CAR-T therapy companies worldwide. Read on to learn more about innovative CAR-T cell therapy companies and the technologies they are using to fight cancer. |
|
|
Designer Exosomes: The Future of Targeted Therapy and Regenerative Medicine
In the evolving landscape of biomedical innovation, exosomes have emerged as one of the most promising tools for next-generation therapies. These nanoscale, membrane-bound vesicles, naturally secreted by cells, play a critical role in intercellular communication. Because of their inherent ability to shuttle biomolecules like RNA, proteins, and lipids between cells, exosomes have captivated the attention of researchers and biotech companies for their vast potential in therapeutic applications. |
|
|
[REPORT] Global CAR-T Cell Therapy Market - Market Size, Forecasts, Trials & Trends, 2025
Since 2017, 13 CAR-T cell therapies have reached commercialization. Seven therapies have been approved by the U.S. FDA, after which approvals for them were issued in other healthcare markets as well. These therapies include Kymriah, Yescarta, Tecartus, Breyanzi, Abecma, Carvykti, and Aucatzyl. Beyond the U.S., four CAR-T therapies—Relma-cel, Fucaso, Yuanruida, and Zever-cel—have been approved by China’s NMPA. Additionally, two therapies, NexCAR19 and Qartemi, have received approval from India’s CDSCO. This report reveals market size figures for the Global CAR-T Cell Therapy Market, segmented by Product, Geography, and Indication, with forecasts through 2032. |
|
|
[REPORT] Mesenchymal Stem Cells / Medicinal Signaling Cells (MSCs) – Advances & Applications, 2025
Today, 12 MSC-based products have received regulatory approvals. This includes 11 full approvals and a 12th conditional approval within China. The Republic of Korea has approved five products; Japan and the EU have approved two products; and India, Iran, and Australia have approved one each. Market competitors have also developed 17 biomaterial-based MSCs and MSC progenitor products, which are largely being used for orthopedic indications. Companies like Cynata are pioneering iPSC-derived MSC (iMSC) production technologies, supporting large-scale therapeutic development. In total, at least eight companies are now developing iMSCs. |
|
|
Interested to learn about other market segments, such as MSCs, iPSCs, CAR-T cells, exosomes, cord blood and tissue, or the cell therapy industry at large?
Explore the BioInformant Shop. |
|
|
*Want to advertise in this newsletter? Contact us at Info@BioInformant.com. |
 |
|
|
|
|
|
|